
(colored violet above) are also exceptionally reactive, but for the opposite reason. In their chemical reactions halogen atoms achieve a valence shell octet by capturing or borrowing the eighth electron from another atom or molecule. The halogens (F, Cl, Br etc.) are one electron short of a valence shell octet, and are among the most reactive of the elements (they are colored red in this periodic table). In the periodic table above these elements are colored beige. This group of inert (or noble) gases also includes krypton (Kr: 4s 2, 4p 6), xenon (Xe: 5s 2, 5p 6) and radon (Rn: 6s 2, 6p 6). The other members of group 8 have a characteristic valence shell electron octet (ns 2 + np x 2 + np y 2 + np z 2). Helium is unique since its valence shell consists of a single s-orbital. For example, helium, neon and argon are exceptionally stable and unreactive monoatomic gases.
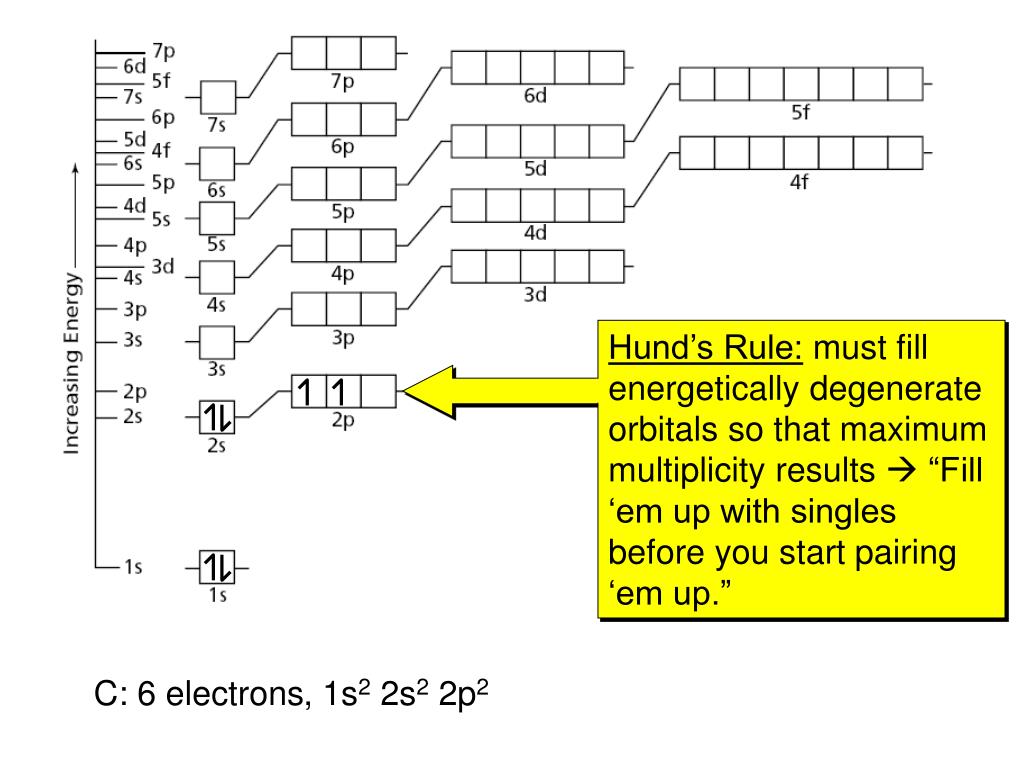
The chemical properties of the elements reflect their electron configurations. The highest occupied electron shell is called the valence shell, and the electrons occupying this shell are called valence electrons. In the third period of the table, the atoms all have a neon-like core of 10 electrons, and shell #3 is occupied progressively with eight electrons, starting with the 3s-orbital. As we progress from lithium (atomic number=3) to neon (atomic number=10) across the second row or period of the table, all these atoms start with a filled 1s-orbital, and the 2s-orbital is occupied with an electron pair before the 2p-orbitals are filled. Shell #2 has four higher energy orbitals, the 2s-orbital being lower in energy than the three 2p-orbitals. According to the Aufbau principle, the electrons of an atom occupy quantum levels or orbitals starting from the lowest energy level, and proceeding to the highest, with each orbital holding a maximum of two paired electrons (opposite spins).Įlectron shell #1 has the lowest energy and its s-orbital is the first to be filled. The truncated periodic table shown above provides the orbital electronic structure for the first eighteen elements (hydrogen through argon).

Consequently, our understanding of organic chemistry must have, as a foundation, an appreciation of the electronic structure and properties of these elements. Other interactive periodic tables provide comprehensive data for each element, including nuclide properties, environmental and health factors, presentation in different languages and much more.įor comic relief you may wish to examine a periodic table linked to element references in comic books.įour elements, hydrogen, carbon, oxygen and nitrogen, are the major components of most organic compounds. There are, of course, over eighty other elements.Ī complete periodic table, having very useful interactive links has been created by Mark Winter. The periodic table shown here is severely truncated. This module introduces some basic facts and principles that are needed for a discussion of organic molecules.Įlectron Configurations in the Periodic Table 1A The study of organic chemistry must at some point extend to the molecular level, for the physical and chemical properties of a substance are ultimately explained in terms of the structure and bonding of molecules. Electron Configurations & The Periodic Table


 0 kommentar(er)
0 kommentar(er)
